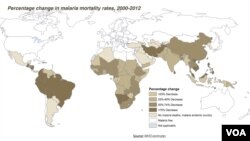
Scientists are developing a vaccine against malaria that is designed to limit the illness in children who have been bitten by mosquitoes carrying the disease-causing organism. They have discovered a protein that is essential for malaria parasites to cause severe illness.
With the protein, called SEA, the parasite is able to burst through infected red blood cells, ramping up disease symptoms. But malaria parasites deprived of SEA are trapped inside the cells where they wither away and are eventually eliminated from the body by the spleen.
Researchers at Brown University in Providence, Rhode Island, and Boston Children’s Hospital in Massachusetts discovered the protein.
Jonathan Kurtis is director of the Center for International Health Research at Rhode Island Hospital and lead author of the study which appears in the journal Science.
Kurtis says an experimental vaccine containing antibodies against the parasitic protein was developed and tested in mice. Rodents that received the vaccine were only mildly sick and had fewer parasites in their bodies than untreated mice.
Next, Kurtis says investigators measured levels of antibodies to the SEA protein in a group of 785 Tanzanian children.
“And children with antibodies to our protein never got severe malaria - there were zero cases - as compared to children who did not have antibodies to our protein,” Kurtis said.
Researchers then analyzed blood samples collected in 1997 from 140 children in Kenya. Investigators found there were 50 percent fewer parasites in the serum of youngsters that produced antibodies to SEA during a high transmission season. As with the Tanzanian children, there were also no severe cases of the disease in children with antibodies against the protein, according to Kurtis.
“And so the thought is by immunizing people with the SEA proteins, so that they make their own antibodies, they’ll be protected,” he added.
The next step, Kurtis says, is to test a laboratory-made SEA vaccine to see whether it works in primates.
Kurtis says he’s excited that researchers may be on the verge of an effective drug that lessens the severity of a malaria infection, but he’s also humbled.
“Our eye has to be on the prize. And the prize is - you’ve got a child every 15 seconds, just during this phone conversation, you know dozens and dozens of children have died of malaria,” Kurtis said. "It’s just unbelievable.”
If the vaccine proves to be safe and effective in monkeys within the next year, researchers expect they’ll move quickly to human vaccine trials.
Kurtis says the goal eventually is to immunize youngsters in malaria-endemic regions at the same time they are vaccinated against other childhood illnesses.
With the protein, called SEA, the parasite is able to burst through infected red blood cells, ramping up disease symptoms. But malaria parasites deprived of SEA are trapped inside the cells where they wither away and are eventually eliminated from the body by the spleen.
Researchers at Brown University in Providence, Rhode Island, and Boston Children’s Hospital in Massachusetts discovered the protein.
Jonathan Kurtis is director of the Center for International Health Research at Rhode Island Hospital and lead author of the study which appears in the journal Science.
Kurtis says an experimental vaccine containing antibodies against the parasitic protein was developed and tested in mice. Rodents that received the vaccine were only mildly sick and had fewer parasites in their bodies than untreated mice.
Next, Kurtis says investigators measured levels of antibodies to the SEA protein in a group of 785 Tanzanian children.
“And children with antibodies to our protein never got severe malaria - there were zero cases - as compared to children who did not have antibodies to our protein,” Kurtis said.
Researchers then analyzed blood samples collected in 1997 from 140 children in Kenya. Investigators found there were 50 percent fewer parasites in the serum of youngsters that produced antibodies to SEA during a high transmission season. As with the Tanzanian children, there were also no severe cases of the disease in children with antibodies against the protein, according to Kurtis.
“And so the thought is by immunizing people with the SEA proteins, so that they make their own antibodies, they’ll be protected,” he added.
The next step, Kurtis says, is to test a laboratory-made SEA vaccine to see whether it works in primates.
Kurtis says he’s excited that researchers may be on the verge of an effective drug that lessens the severity of a malaria infection, but he’s also humbled.
“Our eye has to be on the prize. And the prize is - you’ve got a child every 15 seconds, just during this phone conversation, you know dozens and dozens of children have died of malaria,” Kurtis said. "It’s just unbelievable.”
If the vaccine proves to be safe and effective in monkeys within the next year, researchers expect they’ll move quickly to human vaccine trials.
Kurtis says the goal eventually is to immunize youngsters in malaria-endemic regions at the same time they are vaccinated against other childhood illnesses.