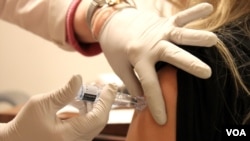
Researchers have developed a gene therapy against pandemic influenza in laboratory animals, one that stops infection at the point of entry - the nose. The therapy could potentially thwart the most aggressive viral pathogens, saving the lives of an estimated 500,000 people who die worldwide each year from the flu.
The genetic therapy developed by researchers at the University of Pennsylvania expresses so-called broadly neutralizing antibodies, giving lab mice and ferrets almost complete protection against a number of lethal avian influenza strains, including those isolated from the deadly 1918 and 2009 pandemics.
Unlike conventional vaccines which stimulate the body’s natural immune system to fight an infection, broadly neutralizing antibodies halt a virus’s biological activity so it cannot make people sick by infecting cells in the first place. The antibodies can become effective in two to three days.
The head of the University of Pennsylvania's Gene Therapy Program, James Wilson, says scientists created a nasal spray to introduce protective genes.
“And create what I call a "bioshield" around the nose and the mouth to prevent the influenza virus from replicating,” Wilson said.
The genes, which engineer the tissue to produce protective antibodies, were delivered by a harmless cold virus in the nasal spray.
The therapy uses a single gene that produces antibodies against many different flu strains, hence the term, "broadly neutralizing antibodies." Wilson says this broad-based strategy protected all mice exposed to lethal amounts of three strains of H5N1 and two strains of H1N1.
But the microbes replicated or reproduced rapidly in untreated rodents. The nasal spray was also successfully tested in ferrets, a good model for human flu because the tiny, furry animals cough and sneeze when sick.
Conventional vaccines to protect against seasonal influenza are not 100 percent effective in preventing illness. The viral strains mutate rapidly, so there is little or no immune-system protection stimulated by the previous year’s flu shot, and the pathogens can evade experts' predictions of what virus is likely to be in circulation during the coming flu season.
Wilson says a different approach to flu protection is needed. Researchers are currently in discussions with U.S. drug regulators about quickly testing the therapy in humans using a safe flu strain. According to Wilson, they are aiming to manufacture and stockpile the drug in anticipation of a serious influenza pandemic.
“So then there is a pretty direct path into first, in human safety and then efficacy studies, which we have charted out. And with the right resources, we could move very quickly on that,” Wilson said.
Wilson says the U.S. government has also expressed an interest in using the broadly neutralizing antibody approach to protect against bioweapons, such as anthrax and other toxic agents.
An article on the development of a gene therapy against pandemic influenza is published in Science Translational Medicine.
The genetic therapy developed by researchers at the University of Pennsylvania expresses so-called broadly neutralizing antibodies, giving lab mice and ferrets almost complete protection against a number of lethal avian influenza strains, including those isolated from the deadly 1918 and 2009 pandemics.
Unlike conventional vaccines which stimulate the body’s natural immune system to fight an infection, broadly neutralizing antibodies halt a virus’s biological activity so it cannot make people sick by infecting cells in the first place. The antibodies can become effective in two to three days.
The head of the University of Pennsylvania's Gene Therapy Program, James Wilson, says scientists created a nasal spray to introduce protective genes.
“And create what I call a "bioshield" around the nose and the mouth to prevent the influenza virus from replicating,” Wilson said.
The genes, which engineer the tissue to produce protective antibodies, were delivered by a harmless cold virus in the nasal spray.
The therapy uses a single gene that produces antibodies against many different flu strains, hence the term, "broadly neutralizing antibodies." Wilson says this broad-based strategy protected all mice exposed to lethal amounts of three strains of H5N1 and two strains of H1N1.
But the microbes replicated or reproduced rapidly in untreated rodents. The nasal spray was also successfully tested in ferrets, a good model for human flu because the tiny, furry animals cough and sneeze when sick.
Conventional vaccines to protect against seasonal influenza are not 100 percent effective in preventing illness. The viral strains mutate rapidly, so there is little or no immune-system protection stimulated by the previous year’s flu shot, and the pathogens can evade experts' predictions of what virus is likely to be in circulation during the coming flu season.
Wilson says a different approach to flu protection is needed. Researchers are currently in discussions with U.S. drug regulators about quickly testing the therapy in humans using a safe flu strain. According to Wilson, they are aiming to manufacture and stockpile the drug in anticipation of a serious influenza pandemic.
“So then there is a pretty direct path into first, in human safety and then efficacy studies, which we have charted out. And with the right resources, we could move very quickly on that,” Wilson said.
Wilson says the U.S. government has also expressed an interest in using the broadly neutralizing antibody approach to protect against bioweapons, such as anthrax and other toxic agents.
An article on the development of a gene therapy against pandemic influenza is published in Science Translational Medicine.