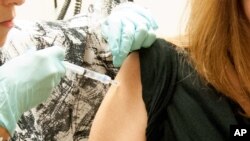
Two experimental Ebola vaccines, and one against the related Marburg virus, have proven to be safe and effective in generating an immune response against the deadly infections.
The findings are from the first vaccine trial against the microbes, known as filoviruses, in Africa.
A study announcing the results, conducted in Kampala, Uganda, is published in the journal The Lancet.
The vaccines were given over the course of two months to 108 healthy volunteers, who were randomly assigned to receive either the Ebola vaccine, the Marburg vaccine, both compounds or a placebo.
The DNA-based drug, which contains genetic information from the Ebola and Marburg viruses, stimulated the formation of neutralizing antibodies and T-cells against both pathogens.
The immune responses were seen four weeks after the third injection in 57 percent of those who received an Ebola Zaire vaccine and in almost half of those participants given the Ebola and Marburg vaccines.
However, the response did not last long. The antibodies produced by the vaccines returned to undetectable levels in the bloodstream within 11 months of vaccination.
Still, the vaccines were well-tolerated and are seen as a forerunner to a more potent compound, called the NIAID/GSK vaccine, currently in clinical trials in the U.S., U.K., Mali and Uganda. The West African nations are the epicenter of the Ebola epidemic, which has infected more than 20,000 people, killing more than 7,500 patients so far.
The vaccine against Ebola uses a chimpanzee adenovirus or cold virus to carry segments of the Ebola gene into the body. The chimp virus is used because most people have developed immunity against human adenovirus.
The study's lead author, Julie Ledgerwood of the National Institutes of Allergy and Infectious Diseases, said the findings in Africa are encouraging because that's where the risk of Ebola infection is greatest.
Larger trials of NIAID/GSK around the world are planned for early 2015.