A new microscope that can capture 3-D images in real time may lead to a better understanding of the inner workings of life.
For more than a century, scientists thought they had reached the limit of what they could see under an optical microscope. They were wrong. The 2014 Nobel Prize in Chemistry was awarded for work that lets researchers observe a single molecule - 100 times smaller than what can be seen with a regular microscope.
Eric Betzig was among the scientists awarded this year's Nobel Prize for Chemistry. He’s a physicist, inventor and engineer based at the Howard Hughes Medical Institute in Ashburn, Virginia. He says the microscope that led to the Nobel is good for high resolution imagery, but not so good for looking at cells that are moving very rapidly or that are very fragile under light.
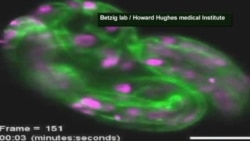
Eric Betzig has developed a new microscope that captures 3-D images of subcellular activity in real time:
So he began research in a different direction. “I shifted my attention to trying to develop a microscope that would be as transformative in terms of its ability to see fast dynamics, without damaging the cell, as the earlier super resolution microscopes were transformative for looking at the structure in high resolution inside the cell.”
Betzig first tried using light beams that could be shaped in different ways. He turned them into long thin pencils of light, not unlike the technology used to scan barcodes in a supermarket. But that light was too bright and damaged the cell as it swept along. So he turned to an ultrathin sheet of light.
“It does absolutely no damage to the cell. And you can follow it very quickly in 3-D. So you are looking at the 3-D dynamics of these cells, up to several volumes a second or on a plane-by-plane basis up to 1,000 planes a second through the cell.”
Writing in the journal Science, Betzig describes his work examining 20 distinct biological systems, including embryonic development in nematodes and fruit flies. He says using this approach, scientists can trace nerve pathways that form synapses in the brain, watch the activity of a fertilized egg, chart the progress of proteins that clump together to cause disease, or follow infection-fighting T-cells. “We’ve been able to see how T-cells interact with other cells in exquisite 4-dimensional detail, three dimensions and then the movie of what’s happening of that going on.”
Betzig says the advances allow scientists to image cell, tissue and organ formation in all their complexity at small scale and high resolution. “We now have, with this, a tool that can relate these molecular signals that then basically provide the orchestration for how cells divide and form new organisms. So, we’ve taken it essentially from that singular molecule level and connected it all the way up to multi-cellular systems and how they develop.”
Betzig is hopeful that this microscope will make it easier to understand the normal functioning of the cell and what happens at the subcellular level to create disease.





![[mzalewski] 3D Microscope 2 Web [mzalewski] 3D Microscope 2 Web](https://gdb.voanews.com/b2033e58-357b-4912-92cc-19cabc5f07b6_tv_w250_r1.jpg)
