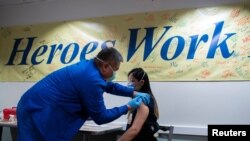
President-elect Joe Biden announced a controversial shift in COVID-19 vaccination strategy Friday, aiming to release all available doses as soon as possible.
Currently, half the doses of the two-shot vaccines are being reserved so that everyone who gets a first shot is guaranteed a second shot in three to four weeks.
Biden "believes the government should stop holding back vaccine supply so we can get more shots in Americans’ arms now," said Biden transition spokesman T.J. Ducklo in a statement.
Given short supplies, second doses may be delayed.
The U.S. Food and Drug Administration does not approve.
"Making such changes that are not supported by adequate scientific evidence may ultimately be counterproductive to public health," the FDA said in a statement Monday.
Anthony Fauci, head of the National Institute of Allergy and Infectious Diseases, told CNN last week that he opposed delaying second doses.
“I would not be in favor of that,” he said. “We’re going to keep doing what we’re doing.”
Fauci's statement followed the United Kingdom's decision in late December to accelerate delivery of first doses and delay second doses by up to 12 weeks. France is using a six-week schedule.
The U.K. is on lockdown as cases spike. A coronavirus variant that appears to spread more rapidly may be to blame. That variant has turned up in dozens of countries, including the United States.
'Pragmatic' vs. 'faith-based'
The World Health Organization also gave the nod on Friday to delaying second doses, calling it "a pragmatic approach to maximizing the number of individuals benefiting from a first dose while vaccine supply continues to increase."
In clinical trials, vaccines from Pfizer-BioNTech and Moderna were about 95% effective when patients received their second dose three to four weeks after the first.
WHO said the time between doses may be extended up to six weeks.
One of the biggest objections many scientists have to making major changes to the dosing is that the effects are unknown because they have not been studied.
"That's faith-based decision-making, not evidence- and data-based," said Harvard University immunologist Barry Bloom.
"We have a crisis here," countered his Harvard colleague epidemiologist Marc Lipsitch, "and acting on limited information is what you have to do in a crisis."
Partial protection
The vaccines appear to give some protection after the first shot. Pfizer estimates theirs is about 50% effective. Moderna claims about 80% to 90%. But it's hard to say for sure because the studies were not designed to answer that question.
Though delaying the second, booster dose for a few weeks may not present problems, it's not clear how long protection lasts after the first dose.
"I don't think there's anything magic about 21 or 28 days for a booster," Bloom said, but added, "I don't know how long it is appropriate to hold off."
One risk is that waiting longer between doses might help the virus develop resistance to it, according to Johns Hopkins University immunologist Andrew Pekosz.
When scientists want to see how a virus can become resistant to a vaccine, they attack it with a weakened immune response – much like giving someone only one dose of a two-dose vaccine, he said.
"You don't want to have that experiment being done on a population level by getting a lot of people with just so-so immunity that allows ... for variants to emerge," Pekosz said. "You're much better off getting to that high level of efficacy as fast as possible."
"I don't buy that argument," Harvard's Lipsitch said, adding that unvaccinated people might be a bigger risk.
Another risk is that making changes could undermine public confidence, Bloom said. "For the integrity of the process, which is under question by many people – whether vaccines are safe, whether the government is corrupt, whether FDA knows what it's doing – there's a certain logic to saying, 'We have good evidence for one thing, stick with it,' " he said.
But even the administration of initial shots is going much more slowly than anticipated. While more than 21 million doses had been delivered as of Thursday, just short of 6 million people had received them.
"It seems like the hurdle is getting the needle into people's arms right now," Pekosz said. "If that's the hurdle, then I don't think you're going to buy yourself much benefit" from delaying the second dose.