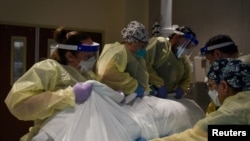
Members of a U.S. Centers for Disease Control and Prevention advisory committee are meeting Tuesday to determine who should get inoculated first against the coronavirus once a vaccine receives final approval.
The CDC’s Advisory Committee for Immunization Practices wants to inform the public about its recommendation before a decision is announced by the U.S. Food and Drug Administration, committee chairman Dr. Jose Romero told CNN on Tuesday.
The FDA is considering an emergency request from Pfizer to authorize the use of its vaccine. Moderna said Monday it also would apply for emergency use authorization of its vaccine.
The CDC has already recommended that front-line health care workers and support personnel receive the first doses. The CDC also said residents of nursing homes and other long-term care facilities should be among the first to receive vaccinations.
Hours after Moderna’s announcement, Health and Human Services Secretary Alex Azar said the agency would announce its decision up to a week after it decides on Pfizer’s application.
Dr. Larry Corey of the University of Washington, who leads vaccine clinical trials in the U.S., has said once Pfizer’s and Moderna’s vaccines are approved, they could make 50 million doses in January.
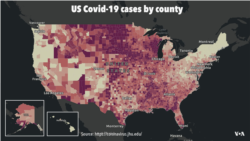
The advisory committee is meeting one day after nearly 139,000 new coronavirus cases and 826 deaths were reported in the U.S., according to Johns Hopkins University.
As it has for months, the U.S. continues to lead the world in coronavirus infections, with more than 13.5 million of the world’s 63.3 million cases. Over 268,000 people have died of COVID-19 in the U.S., more than any other country, according to Johns Hopkins, which reports 1.47 million deaths worldwide.
In Europe, which is also experiencing surges in coronavirus infections and related deaths, BioNTech and Moderna have applied to the European Union for approval of their vaccines, the EU said on Tuesday. EU officials are expected to decide on at least one of the vaccines by the end of December.
BioNTech has already filed a similar application with the FDA. Its vaccine is under review in Australia, Canada, Japan and in other countries.
Since it began nearly a year ago, the coronavirus pandemic has dramatically increased the number of people who are experiencing extreme poverty, according to the United Nations.
The U.N. said in its annual humanitarian report that 235 million people, or one in 33 people, will require basic needs like food, water and sanitation in 2021, a 40% increase from this year.
The report said the greatest need for humanitarian assistance next year is in Afghanistan, Syria, Yemen, the Democratic Republic of Congo and Ethiopia.
The U.N. contributed a record $17 billion in 2020 for humanitarian response worldwide, the report said.