The first new guidelines for diagnosing Alzheimer's disease in almost 30 years will help doctors identify people in the earliest stages of the disease, before symptoms appear.
The guidelines recognize three distinct phases of the fatal brain disorder. In 1984, experts defined Alzheimer's in terms of a devastating decline in memory, thinking, and other brain functions.
But in the decades since, scientists have learned much more about the disease - most notably, that Alzheimer's really starts years earlier, before any symptoms are noticed. In this so-called pre-clinical stage, the brain is undergoing changes that can be identified with MRI scans or other tests.
As the disease progresses, symptoms start to appear. This second stage is called mild cognitive impairment.
"So this is a phase of the disease where patients do indeed have clinical symptoms, generally beginning with memory impairment," says Mayo Clinic researcher Clifford Jack. "The impairment here, though, is not severe enough to prevent the patients from adequately carrying out activities of daily living."
The third stage of the disease is full-blown Alzheimer's.
Jack is the lead author of one of several papers describing and discussing the new diagnosis guidelines in the journal Alzheimer's & Dementia. He says the new guidelines will have no immediate impact on how Alzheimer's patients are treated. Most of the current treatments don't do much good anyway but the new criteria could prove important in the search for therapies that do work.
"Because what these criteria do is formally codify diagnostic criteria that can be employed to identify subjects for inclusion in clinical trials. And the important part here is that the clinical trials need to be targeted to people who are in the very earliest stages of the disease."
According to Jack, many researchers believe that Alzheimer's drugs are largely ineffective because they are given after patients have already lost memory and cognitive skills. The new guidelines will help scientists identify people before symptoms appear, allowing them to be enrolled in studies to see if drugs can affect the course of Alzheimer's if taken early enough.
The Alzheimer's guidelines were developed jointly by the U.S. National Institute on Aging and the Alzheimer's Association, a non-governmental research and advocacy group.
New Alzheimer's Guidelines Focus on Early Diagnosis
- By Art Chimes
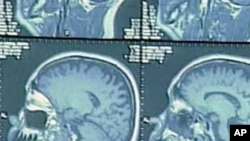
Identifying fatal brain disease early could help lead to new treatments



